Do you even know how big an atom is? It's not easy to answer this IGCSE/O Level Chemistry question because atoms are so tiny that we can't see them with our eyes. Even if we could, we would find that their size is not constant or clear-cut.
Basics of an Atom:
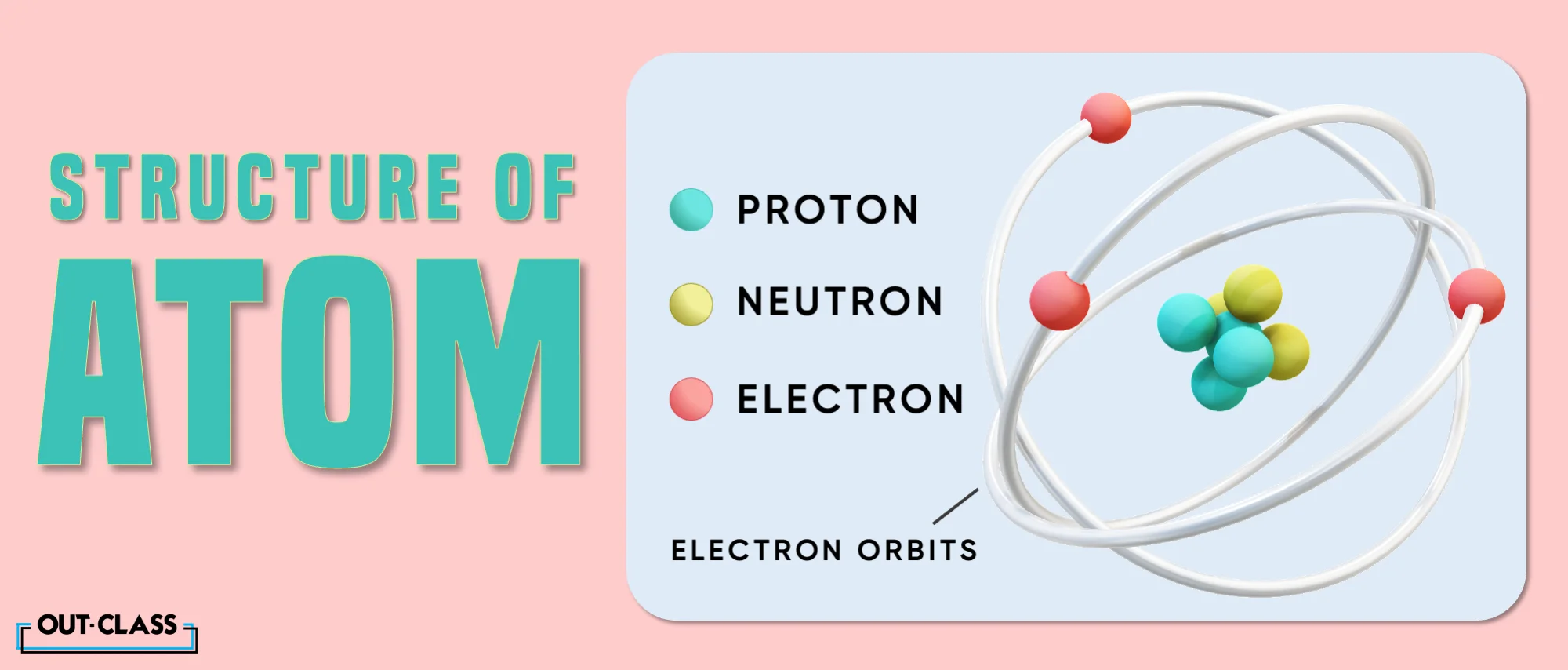
To explain in just one sentence: an atom is the smallest unit of matter, consisting of a nucleus at its centre, composed of protons and neutrons, surrounded by electrons orbiting in energy levels. Well, that's four things but one sentence.
Since their electrons are always moving around the nucleus, in a fuzzy cloud. Well, the very size of this cloud depends on what kind of atom it is and what it is doing. This is also very crucial to the atomic size definition.
Again, what is atomic size and then what is atomic radius size? How can we measure the size of an atom?
Understanding Atomic Size:
Well, there are different methods that we can use, depending on how we define the size. For example, we can use:
-
Atomic radius: This is just half the distance between the centres of two atoms that are next to each other in a molecule or a crystal.
-
Van der Waals radius: This is half the shortest distance between the centres of two atoms which are not connected by any bonds.
-
Ionic radius: This is half the distance between the centres of two ions that have opposite charges. And they are stuck together in an ionic compound.
-
Covalent radius: This is half the distance between the centres of two atoms that share a pair of electrons but in a covalent bond.
-
Metallic radius: This is half the distance between the centres of two metal atoms that are touching each other in a metallic bond, as obvious by the name.
But these methods don't always give us the same answer for the same atom, this depends on their bonds. For instance, carbon has a different atomic radius in diamond (a covalent crystal) than in graphite (a metallic crystal). So, the bond is what determines the size of an atom.
Atomic Size on the Periodic Table
While studying atomic size definition, it's of course crucial to place it on the periodic table itself too.
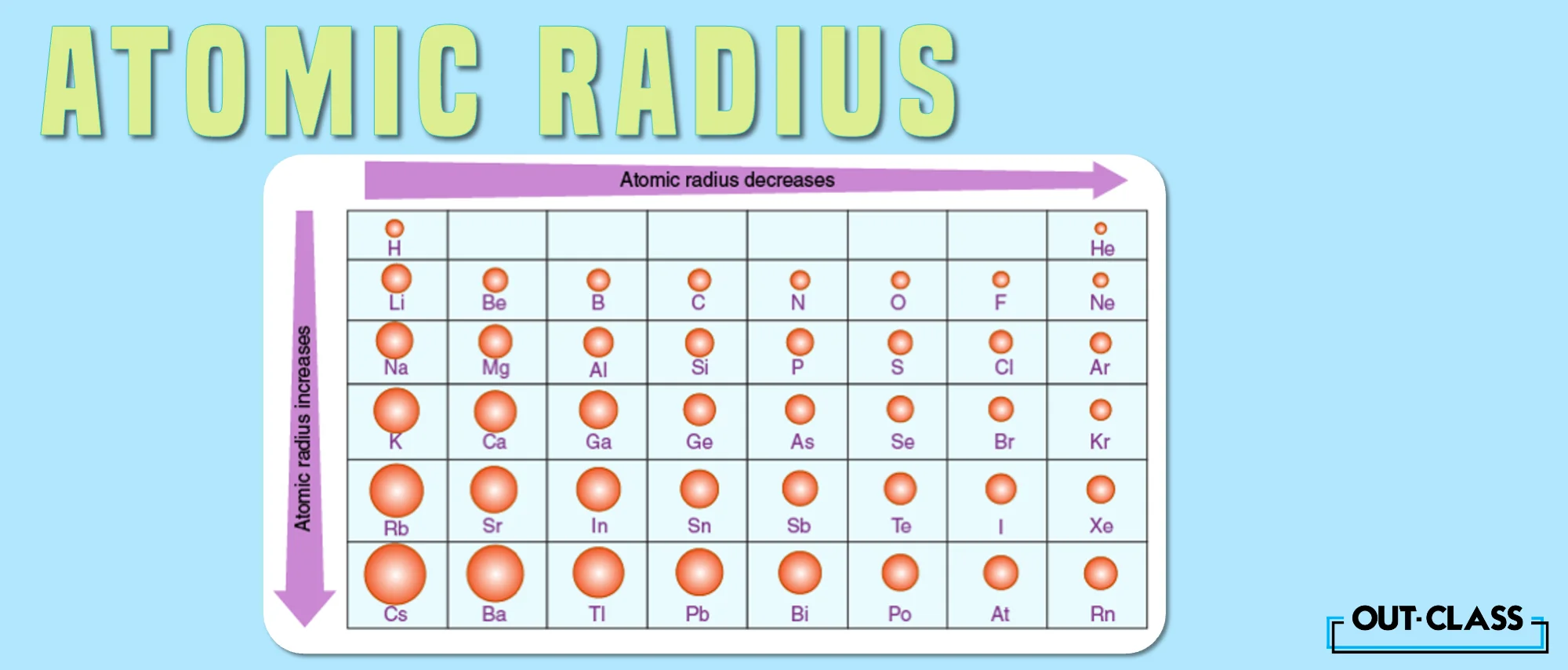
The size of an atom too depends on how many protons and electrons it has and how they are arranged. Generally, atoms get smaller as we move from left to right in a row of the periodic table, as they have more protons but the same number of shells. This makes the nucleus pull the electrons closer because again more protons.
On the other hand, atoms get bigger as we move from top to bottom in a column of the periodic table, because they have more shells but not much more protons. This makes the nucleus pull the electrons weaker.
Interactions with other Atoms
The size of an atom changes when it forms bonds or interactions with other atoms. For example, ions have different sizes than their neutral atoms, depending on their charge and how many neighbours they have. Another factor that explains atomic size.
Usually, positive ions are smaller and negative ions are bigger than their neutral atoms, as they lose or gain electrons and shells.
Wrapping Up
But why does the atomic size definition matter? The size of an atom affects many things, such as how dense, hot, soluble, reactive and electronegative it is. By learning the atomic size definition, how the atomic size works and how it changes in different situations, you can understand more about the amazing world of atoms.