Need help to understand how to calculate ion charges for IGCSE & O Level Chemistry? Look no further! This easy-to-follow guide is here to simplify the concept of finding charges of ions. Let's get a clear understanding of how to calculate the charge on an ion together!
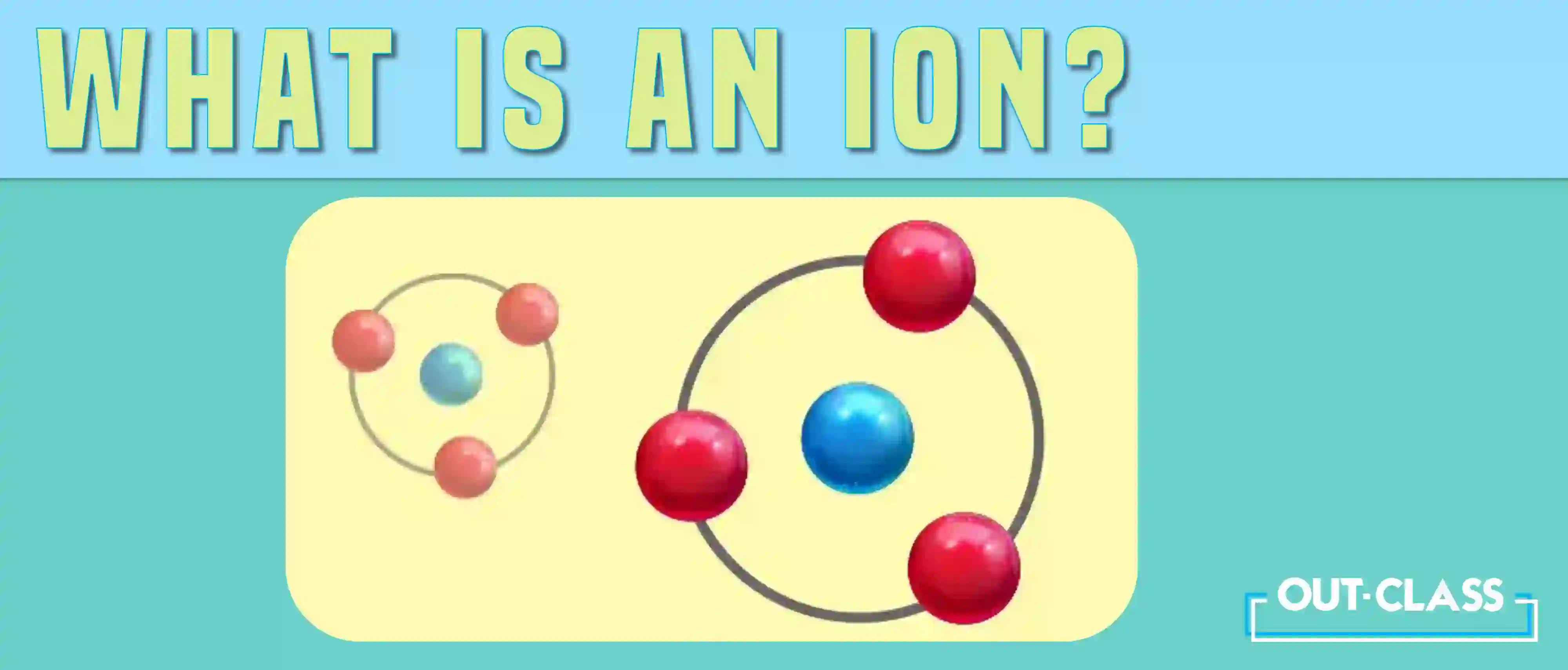
What is an Ion?
The term “ion” comes from the Greek word for ‘going’ and was first used by English chemist and physicist Michael Faraday in 1834 to describe the process of electrolysis. An ion is formed when an atom becomes electrically charged due to either the loss or gain of electrons. This can happen due to chemical reactions or external influences.
Usually, this process occurs so that the atom can gain a full outer shell of electrons, a more stable arrangement of electrons. When an atom gains electrons, it becomes negatively charged, called an anion. When an atom loses electrons, it becomes positively charged, called a cation.
Are Ions Charged?
Yes, ions are charged particles. They form when atoms gain or lose electrons. Losing electrons results in a positive charge; gaining electrons leads to a negative charge.
Calculating the Charge on Single-Atom Ions
Here’s how you can predict the charge on common single-atom ions. Start by looking at the periodic table position:
-
Group 1 elements form ions with a 1+ charge.
-
Group 2 elements form ions with a 2+ charge.
-
Non-metals in Group 17 (or 7) typically form ions with a 1- charge, and Group 16 (or 6) with a 2- charge.
As you can see, what determines the charge of an ion is its position in the periodic table. But why is this? Actually, the charge of an ion depends on how many electrons the atom needs to gain or lose to get a complete outer shell. Naturally, the periodic table group number helps because it tells us how many electrons are in the outer shell of an atom.
Ions with a 2+ Charge Example
Calcium (Ca) in Group 2 loses two electrons, forming Ca2+, and hence obtaining a stable octet in the outer shell.
Charge of Common Polyatomic Ions
-
Ammonium (NH4+)
-
Nitrate (NO3-)
-
Sulfate (SO4 2-)
-
Hydroxide (OH-)
-
Carbonate (CO32-)
-
Phosphate (PO4 3-)
-
Bicarbonate (or Hydrogen carbonate) (HCO3-)
-
Nitrite (NO2-)
-
Sulfite (SO3 2-)
-
Chlorate (ClO3-)
Step-by-Step Approach to Calculate Charge on an Ion
Steps to calculate the charge on an ion include:
-
Identify if the ion is a single-atom or a polyatomic ion.
-
For single-atom ions, refer to the periodic table:
-
Metals usually have positive charges.
-
Non-metals usually have negative charges.
-
For polyatomic ions, learn the common charges (e.g., SO42- has a 2- charge).
Conclusion: How to Determine Charge on Ion
In summary, you can usually find the charge of an ion by looking at its position in the periodic table. Unfortunately, for polyatomic ions, you will have to memorize the common ones which we have listed above.
We here at Out-Class work with students and know that IGCSE & O Level Chemistry can be daunting to approach at first. Luckily, we have curated a crash course that covers all the most important concepts in a structured manner so you can follow along. Past paper practice is also included so that you know how to apply concepts. Check it out.
FAQs:
Q. How are ions charged?
Ions are charged particles formed when atoms gain or lose electrons. Losing electrons results in a positive charge, while gaining electrons leads to a negative charge.
Q. What determines the charge of an ion?
The charge of an ion depends on how many electrons the atom needs to gain or lose to achieve a complete outer shell. This is often indicated by the atom's position in the periodic table.
Q. How can I predict the charge on single-atom ions?
For single-atom ions, refer to the periodic table:
- Group 1 elements form ions with a 1+ charge.
- Group 2 elements form ions with a 2+ charge.
- Nonmetals in Group 17 (or 7) typically form ions with a 1- charge, and Group 16 (or 6) with a 2- charge.
Q. Can you provide an example of an ion with a 2+ charge?
Calcium (Ca) in Group 2 loses two electrons to form Ca2+ and achieve a stable octet in its outer shell.
Q. What are common polyatomic ions and their charges?
Some common polyatomic ions and their charges include:
- Ammonium (NH4+)
- Nitrate (NO3-)
- Sulfate (SO4 2-)
- Hydroxide (OH-)
- Carbonate (CO32-)
- Phosphate (PO4 3-)
- Bicarbonate (HCO3-)
- Nitrite (NO2-)
- Sulfite (SO3 2-)
- Chlorate (ClO3-)
Q. How can I determine the charge on an ion step-by-step?
Identify if the ion is a single-atom or polyatomic ion.
For single-atom ions, refer to the periodic table to determine the charge based on the element's group number.
For polyatomic ions, memorize the common charges associated with each ion.