In this O Level and IGCSE Chemistry ATP blog, we'll unravel the intricacies surrounding the methods of collecting gases, exploring solubility rules, the ideal gas law, and the dance of density. Get ready to learn more about the chemical wonders!
Methods of Collecting Gases:
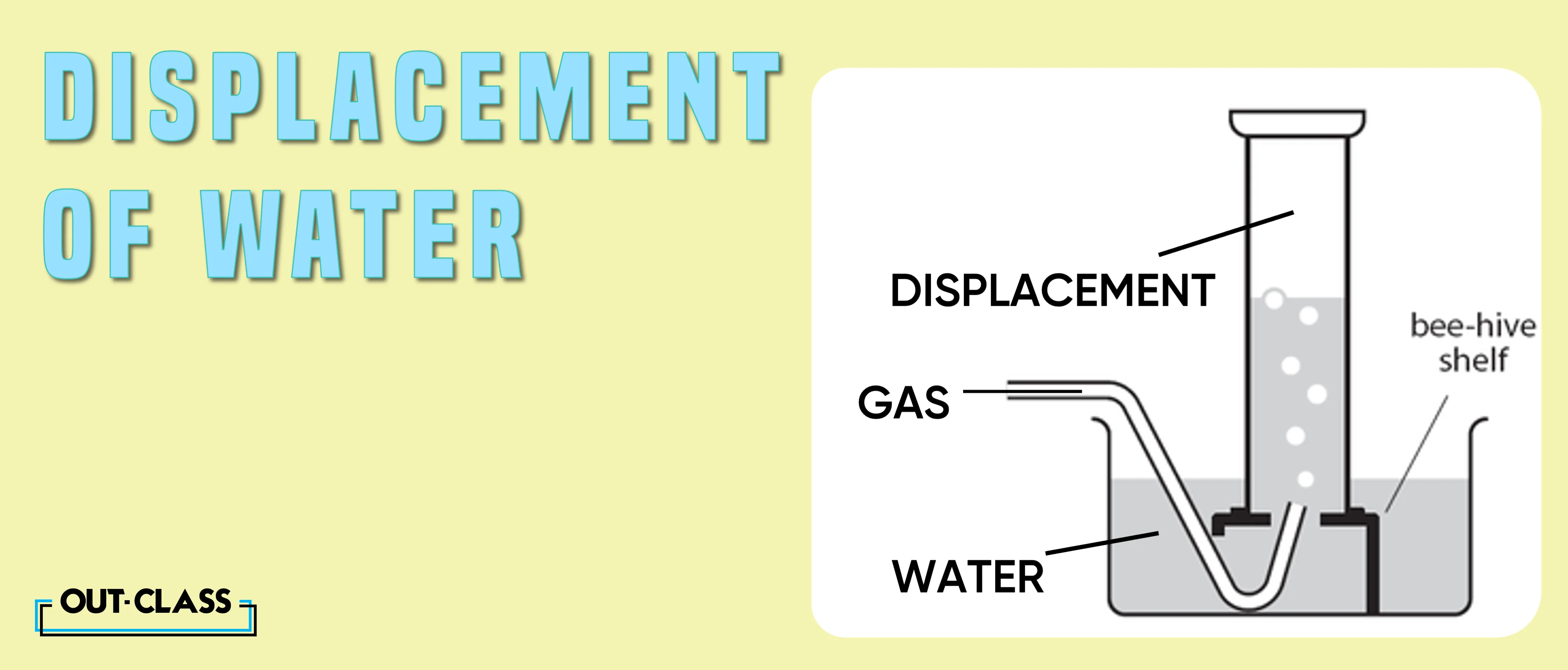
Displacement of Water:
The displacement of water method isn't a one-size-fits-all approach. For insoluble gases like methane, this method becomes the method of choice. As methane doesn't readily dissolve in water, the displacement method ensures accurate and efficient collection.
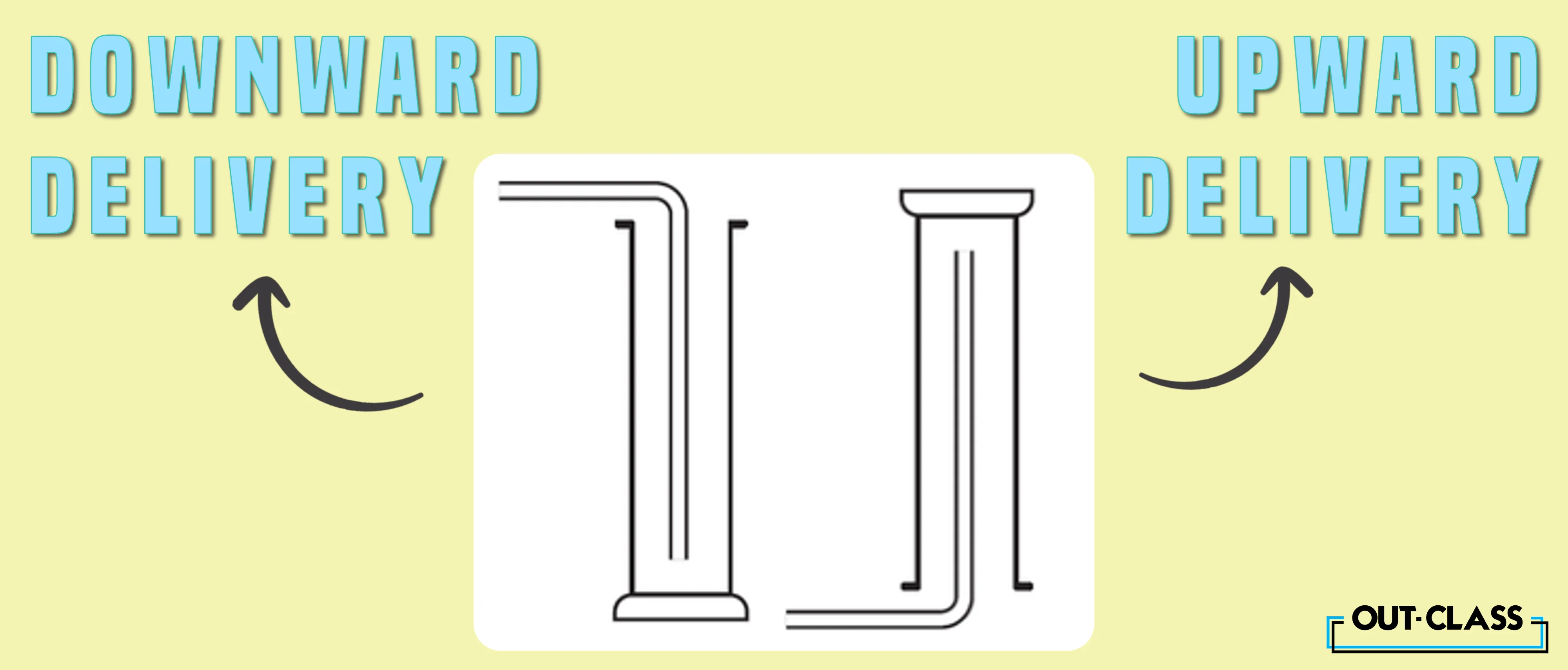
Upward and Downward Delivery:
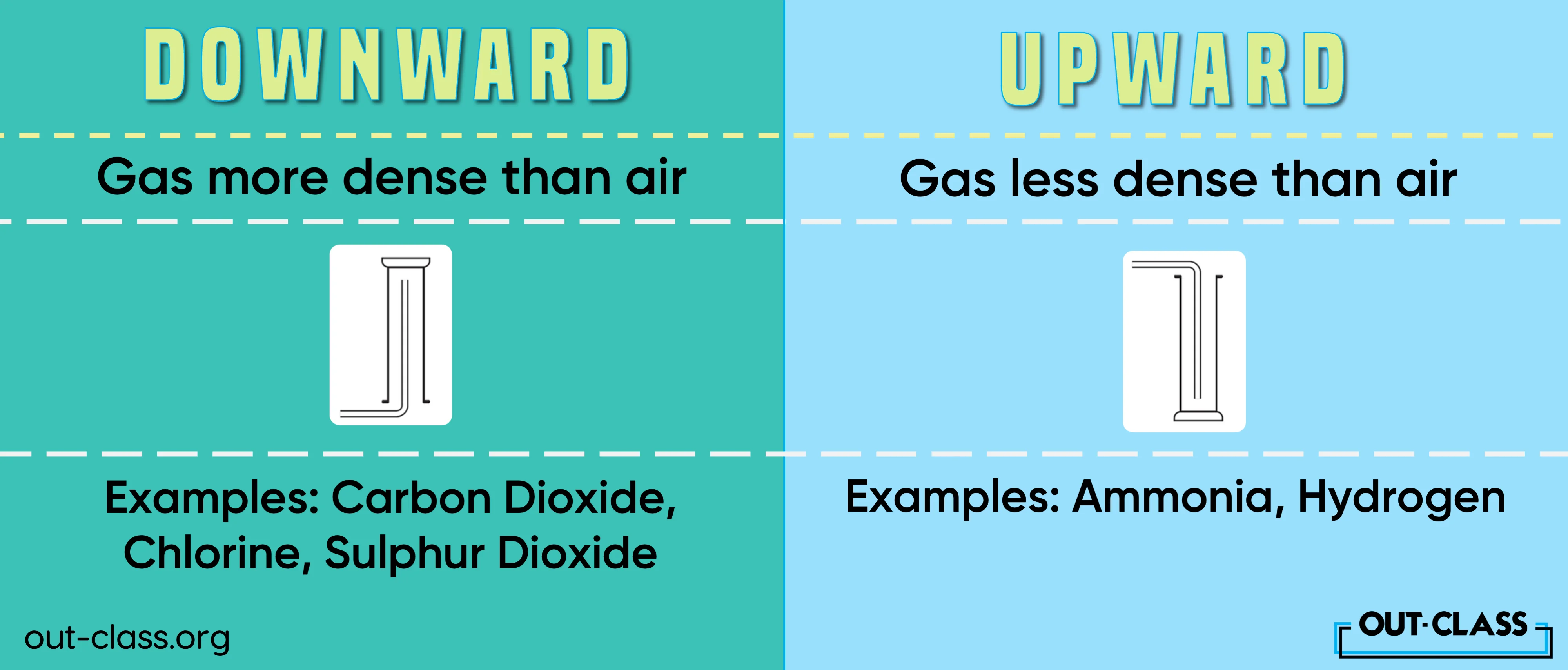
Upward delivery takes centre stage when dealing with gases less dense than air, such as hydrogen and ammonia. On the flip side, downward delivery steals the spotlight for gases more dense than air, exemplified by carbon dioxide. These delivery methods showcase the versatility and precision required in gas collection processes.
Drying Gas:
Ensuring the purity of collected gases is paramount, and drying plays a pivotal role. Silica gel and other desiccants not only remove moisture but also contribute to the reliability of measurements, a critical aspect in any chemistry laboratory.
Gas Solubility in Chemistry:
Factors Affecting Gas Solubility:
In the dynamic world of gas solubility, temperature, pressure, and the nature of the gas and liquid act as conductors orchestrating a symphony of interactions. Understanding these factors provides chemists with the ability to predict and manipulate gas solubility, essential in numerous scientific applications.
How to Calculate Solubility of Gas in Water:
There is a need for precision when calculating the solubility of a gas in water, especially with temperature and pressure. Through mathematical models, scientists can unravel the mysteries of gas solubility, paving the way for advancements in environmental science, pharmaceuticals, and various industrial processes.
Solubility Rules:
Navigating the solubility landscape requires adherence to specific rules. All ammonium salts and group I salts dissolve seamlessly. However, exceptions abound, such as the limited solubility of barium, calcium, and lead sulphate salts. Lead salts, except lead (II) nitrate, are generally insoluble, and carbonate salts follow suit except for group I and ammonium salts. Oxide/hydroxide salts are generally insoluble, except for group I salts, and the solubility of group II oxides/hydroxides increases down the group.
Density of Gases in Chemistry:
How to Find the Density of a Gas in Chemistry:
The density of gases is calculated through mass divided by volume. This calculation provides insights into the physical behaviour of gases under different conditions, a treasure trove for researchers in various scientific domains.
Ideal Gas Law:
The ideal gas law, expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature, is fundamental in understanding gas behaviour. This equation illustrates the dynamic relationship between pressure, volume, temperature, and the quantity of gas.
Conclusion:
In this ultimate ATP chemistry guide, we've navigated the complexities of gas collection methods, explored the enigma of gas solubility, deciphered the language of gas density, and delved into the elegance of the ideal gas law. Armed with this knowledge, students can confidently venture forth into the turbulent waters of the O Level & IGCSE Chemistry ATP.
Still confused? Check Out-Class Crash Courses out! Here's a sneak peek:
FAQs
Q. What are some common methods of collecting gases in Chemistry?
Common methods of collecting gases include displacement of water, upward and downward delivery, and drying gas using desiccants like silica gel.
Q. When is the displacement of water method used in gas collection?
The displacement of water method is used for collecting insoluble gases like methane because they do not readily dissolve in water.
Q. What are upward and downward delivery methods in gas collection?
Upward delivery is used for gases less dense than air, such as hydrogen and ammonia, while downward delivery is used for gases denser than air, like carbon dioxide.
Q. How does drying gas contribute to the reliability of gas collection?
Drying gas using desiccants removes moisture and ensures the purity of collected gases, which is essential for accurate measurements in chemistry experiments.
Q. What factors affect the solubility of gases in Chemistry?
Factors affecting gas solubility include temperature, pressure, and the nature of the gas and liquid, which influence the interactions between gas molecules and the solvent.
Q. How can one calculate the solubility of a gas in water?
The solubility of a gas in water can be calculated using mathematical models that consider temperature, pressure, and other relevant factors affecting gas solubility.
Q. What are some solubility rules to follow in Chemistry?
Solubility rules include the solubility of ammonium salts and group I salts, limited solubility of barium, calcium, and lead salts, insolubility of oxide/hydroxide salts except for group I salts, and other exceptions based on the nature of the salt.
Q. How is the density of a gas calculated in Chemistry?
The density of a gas is calculated by dividing its mass by its volume, providing insights into its physical behaviour under different conditions.
Q. What is the ideal gas law in Chemistry?
The ideal gas law, expressed as PV = nRT, relates the pressure, volume, temperature, and quantity of gas in a closed system and is fundamental in understanding gas behaviour.
Q. How does understanding gas collection methods, gas solubility, and gas density benefit students in O Level & IGCSE Chemistry?
Understanding these concepts helps students perform accurate experiments, predict gas behaviour, and apply theoretical knowledge to practical situations in Chemistry, enhancing their understanding of the subject.