While studying O Level/IGCSE Chemistry, it can be difficult to understand the difference between acid and base, even though they are frequently occurring terms. As such, we here at Out-Class have put together this guide to help you understand the distinction in 5 easy steps.
The five steps are:
Step#4: General Chemistry of Reactions
Step#5: Difference between Acid and Base in tabular form
Let's go further in detail!
5 Differences between Acids and Bases:
#1: Taste and Feel
-
Acids typically taste sour (lemons and vinegar are acidic)
-
Bases tend to taste bitter and have a slippery texture, just like soap
#2: pH Value
-
Acids have a pH value less than 7, whereas bases have a value greater than 7.
-
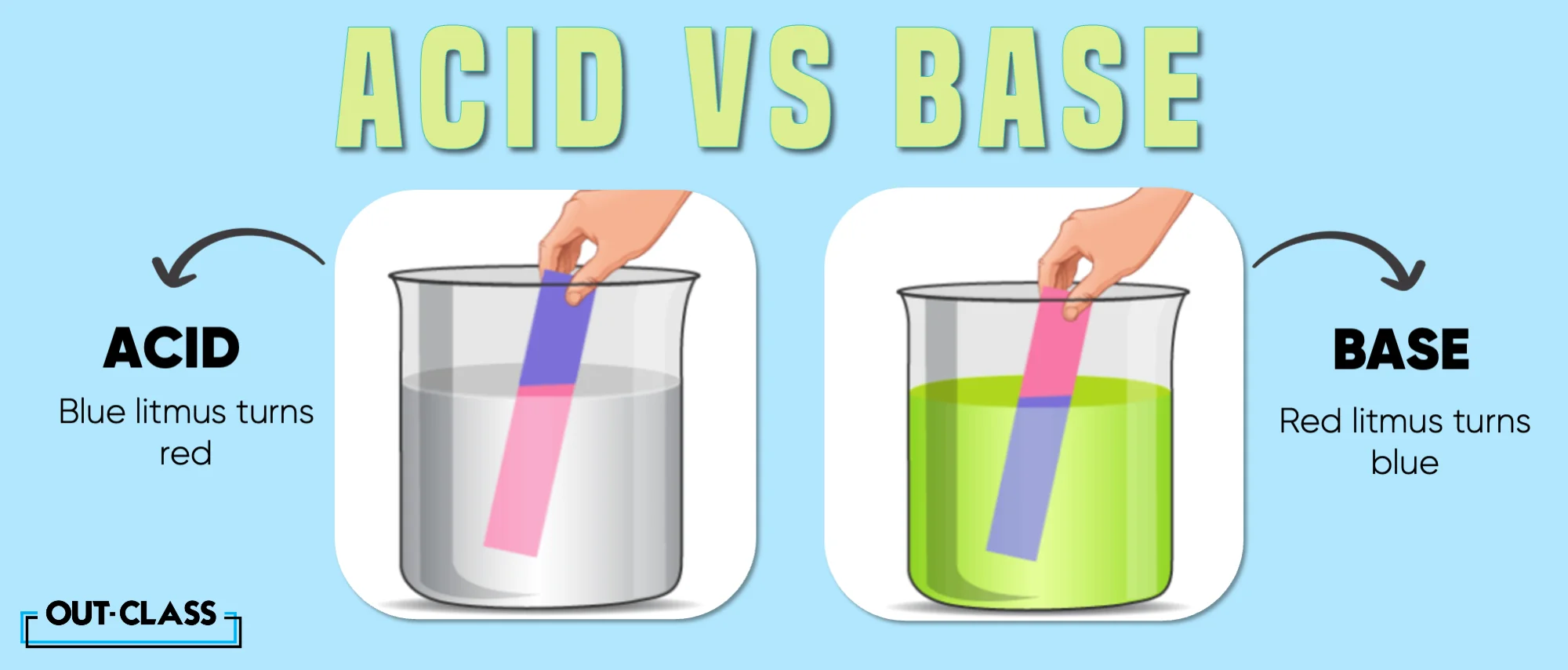
Acids turn blue litmus paper red, whereas bases turn red litmus paper blue.
#3: Reactions with Metals
-
Acids are easily identified since they tend to react with metals, giving off hydrogen gas. The hydrogen gas test is a popular way to identify acids by reacting them with metals.
-
Bases don't typically react with metals.
#4: General Chemistry of Reactions
-
In general, we have:
Acid + Base -> Salt + Water -
Acids donate a proton (H+ ion) to another substance. Bases do the opposite: they can accept a proton. This is the biggest acid-base difference.
#5: Difference between Acid and Base in tabular form
|
Property |
Acids |
Bases |
|
Taste |
Sour |
Bitter |
|
pH Value |
< 7 |
> 7 |
|
Reaction with Metals |
Produces H₂ gas (for metals like magnesium or zinc) |
Typically they do not react; however, some react with metals to produce metal hydroxides and hydrogen gas |
|
Indicator Color Change |
Turns blue litmus red; turns phenolphthalein colourless; turns methyl orange red |
Turns red litmus blue; turns phenolphthalein pink; turns methyl orange yellow |
|
Chemical Structure |
Donates H⁺ ion |
Accepts H⁺ ion |
|
Examples |
Hydrochloric acid (HCl), Sulfuric acid (H₂SO₄) |
Sodium hydroxide (NaOH), Ammonia (NH₃) |
Conclusion
It turns out that the key chemical difference between acid and base is whether they accept or donate protons. This determines all the other physical properties. So the next time you are asked, "What is the difference between acids and bases?", you should be able to answer concisely!
FAQs:
Q. Why do acids taste sour, and bases taste bitter?
The taste of acids is attributed to their hydrogen ion concentration, giving them a sour taste. Bases taste bitter due to their ability to accept protons.
Q. How is the pH value related to acidity or basicity?
The pH value is a measure of the acidity or basicity of a substance. Values less than 7 indicate acidity, while values greater than 7 indicate basicity.
Q. Can litmus paper be used to identify both acids and bases?
Yes, litmus paper is a versatile indicator. It turns red in the presence of acids and blue in the presence of bases.
Q. Why do acids react with metals to produce hydrogen gas?
Acids react with metals due to their ability to donate protons (H+ ions). This reaction releases hydrogen gas as a byproduct.
Q. Do all bases react with metals?
No, not all bases react with metals. While some may react to produce metal hydroxides and hydrogen gas, this is not a universal behaviour for all bases.
Q. How does the chemical structure of acids and bases differ?
Acids donate protons (H+ ions), while bases accept protons. This fundamental difference in their chemical structure governs their reactivity.
Q. Can you provide examples of common acids and bases used in daily life?
Common acids include citric acid (found in citrus fruits) and acetic acid (found in vinegar). Common bases include sodium hydroxide (used in cleaning products) and ammonia.
Q. Where can I find additional resources for O Level/IGCSE Chemistry?
Online educational platforms like Out-Class offer comprehensive resources, including videos and pop quizzes, to enhance your understanding of acids and bases in O Level/IGCSE Chemistry.