Atoms are essential when it comes to the creation of virtually all life on our planet. But what role do protons, electrons – and ions play in life?
Follow us as we explore the differences between an atom and an ion and their properties as mentioned in the IGCSE and O Level Chemistry syllabus.
What is an Atom?
To fully understand the properties of an ion as well as the role it plays in the working and functioning of all matter, we will first need to understand the definition of an atom.
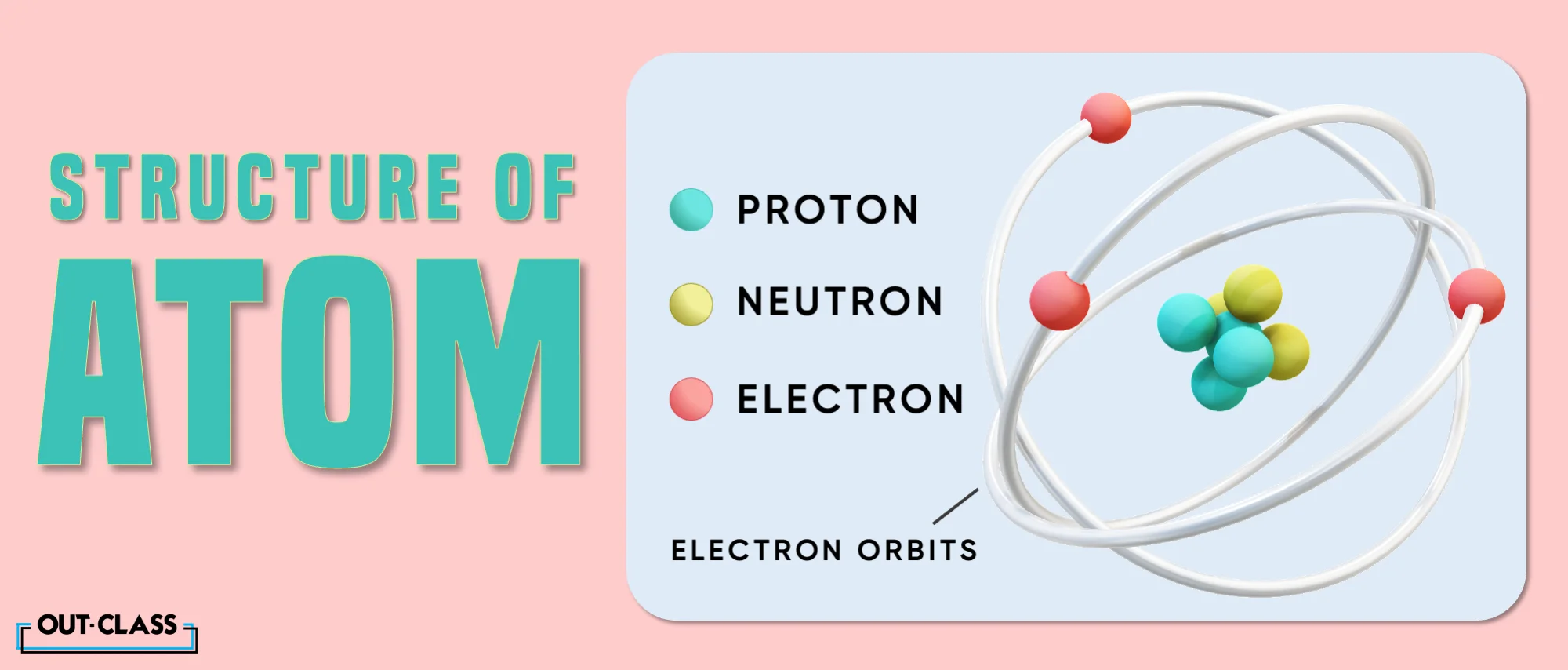
An atom is the basic unit of matter; the building blocks of all life. They are made of three subatomic particles:
-
Protons: Positively charged particles found in the nucleus (center) of the atom. It is like the atom’s core.
-
Neutrons: Neutrally charged particles found in the nucleus of the atom
-
Electrons: Negatively charged particles that go around the nucleus of the atom in specific energy levels or shells.
The number of protons in each atom determines what kind of element it is. This is also referred to as an element’s atomic number.
Examples of Atom:
For example, Hydrogen has one proton, thereby making its atomic number 1 whereas Oxygen has eight protons, making its atomic number 8 on the periodic table. Since atoms generally have an equal number of electrons and protons, they are deemed ‘electrically neutral’; this is because their positive and negative charges cancel each other out.
What is an Ion?
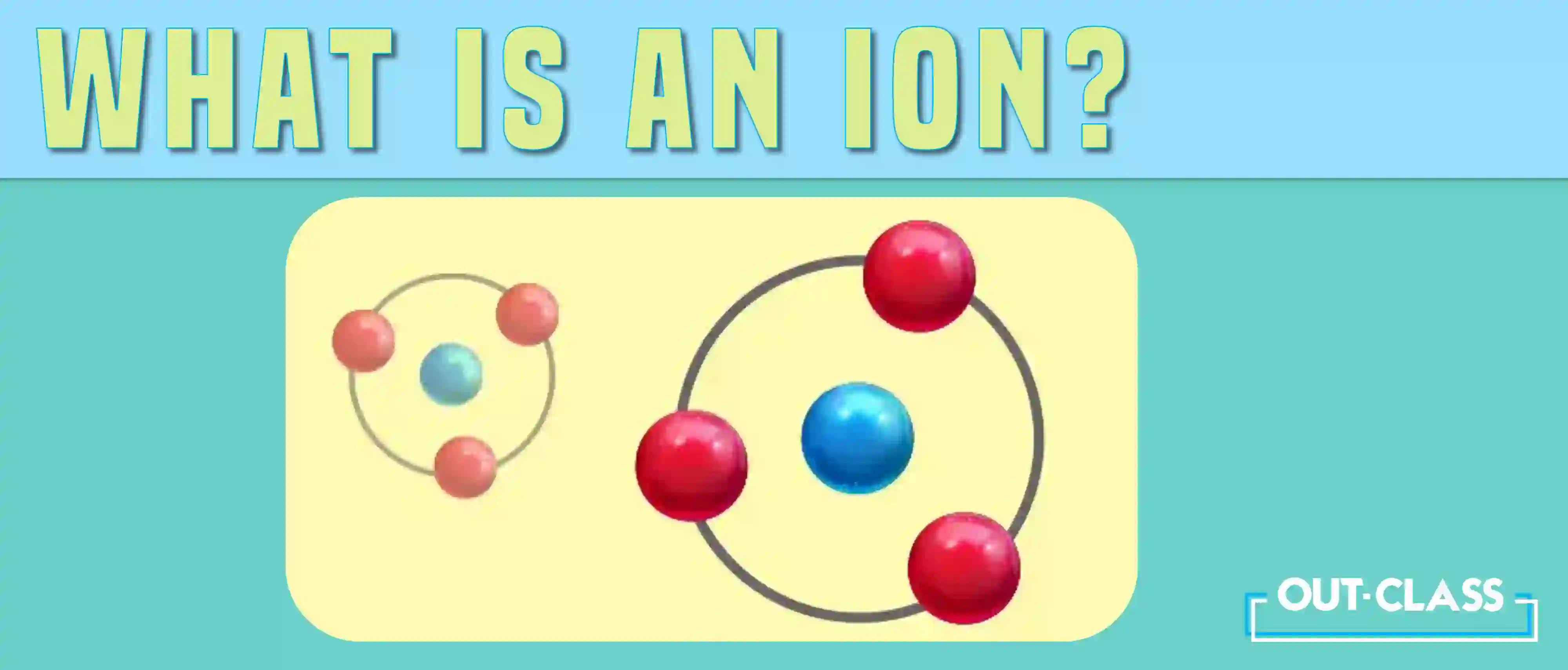
The term “ion” comes from the Greek word for ‘going’ and was first used by English chemist and physicist Michael Faraday in 1834 to describe the process of electrolysis. An ion is formed when an atom becomes electrically charged due to either the loss or gain of electrons. This can happen due to chemical reactions or external influences.
Usually, this process occurs so that the atom can gain a full outer shell of electrons, a more stable arrangement of electrons. When an atom gains electrons, it becomes negatively charged and is called an anion. When an atom loses electrons, it becomes positively charged and is called a cation.
Examples of Ions
One of the most unique things about ions is that they can quickly bond with ions consisting of the opposite charge. Some common compounds are made up entirely of chemically bonded ions. Salt, for example, is made up of a repeating series of chloride anions and sodium cations.
Other examples include electrolytes, such as Chloride, Potassium, Magnesium and Calcium. Electrolytes help to keep the body hydrated while Potassium helps regulate heart and muscle functions. Calcium, as we all know, is very important for bone repair and growth and also plays a role in supporting nerve impulses and blood clotting.
The Key Difference between an Atom and an Ion
How atoms differ from ions is through their properties; atoms are electrically neutral because they have an equal number of protons and electrons. Ions, on the other hand, are electrically charged due to a change in the number of electrons within them. This change ultimately decides how ions will interact in chemical reactions.
Conclusion
To sum up, atoms contain an equal number of protons and ions whereas ions are electrically charged since they contain either a fewer or larger number of protons.
FAQs:
Q. What is an atom and what are its basic components?
An atom is the basic unit of matter, composed of protons, neutrons, and electrons. Protons are positively charged particles in the nucleus, neutrons are neutrally charged, and electrons are negatively charged particles orbiting the nucleus.
Q. How is an element identified based on an atom?
The number of protons in an atom determines the element and is referred to as the atomic number.
Q. What makes an atom electrically neutral?
An atom is electrically neutral when it has an equal number of protons and electrons, balancing positive and negative charges.
Q. What is an ion and how is it formed?
An ion is an electrically charged atom formed by the loss or gain of electrons. When an atom gains electrons, it becomes a negatively charged anion; when it loses electrons, it becomes a positively charged cation.
Related: Difference between Cation and Anion
Q. How do ions interact in chemical reactions?
Ions can quickly bond with ions of the opposite charge. For example, salt is made up of chloride anions and sodium cations.
Related: How to Calculate a Charge on an Ion?
Q. Can you provide examples of ions and their roles in the body?
Examples of ions include chloride, potassium, magnesium, and calcium. These ions play crucial roles in bodily functions, such as regulating heart and muscle functions, supporting nerve impulses, and aiding in bone repair.
Q. What is the key difference between an atom and an ion?
Atoms are electrically neutral, while ions are electrically charged due to a change in the number of electrons.
Q. Where can I learn more about atoms, ions, and IGCSE/O Level Chemistry?
To master the IGCSE/O Level Chemistry curriculum, visit www.out-class.org for comprehensive learning resources.