Hey there, IGCSE and O Level Chemistry students! Have you ever wondered why the ice cubes in your freezer seem to disappear over time, even if you never use them? Or why the scent of a freshly sharpened pencil can be so captivating?
These intriguing phenomena are all around us, and all of them can be explained by a single fascinating scientific process known as sublimation.
What is Sublimation in Science, Anyway?
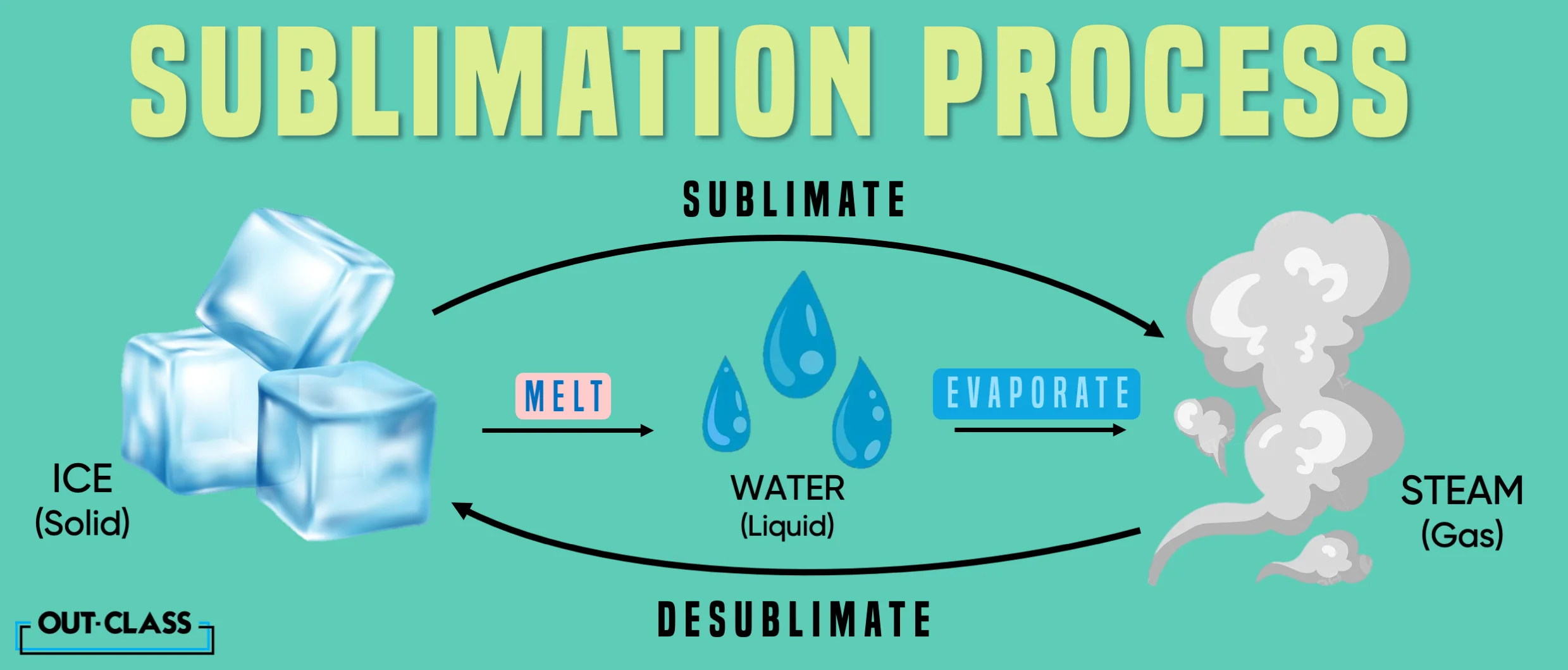
Let's start with the basics of Chemistry. Sublimation, in Science, is the transition of a substance directly from a solid to a gas phase, bypassing the liquid stage.
This occurs when a substance's vapour pressure exceeds its atmospheric pressure, allowing it to transform into a gas without going through the usual melting process.
Enough with the boring theory, let’s dive into some exciting examples of sublimation in daily life!
Sublimation Examples in Daily Life:
Mysterious Ice Cube Disappearance:
Have you ever noticed that ice cubes in your freezer gradually shrink and disappear over time? This is one of the intriguing sublimation examples that occur in daily life. The ice undergoes sublimation, turning directly from a solid into water vapour.

The Pencil Aroma:
Remember the woody fragrance whenever you sharpen a pencil? It's not just any scent; it’s a prime application of sublimation. Those enchanting lead-like scents are a result of sublimation as the lead of the pencil lead directly transforms into a gas, diffusing its distinct aroma into the surroundings.

Air Fresheners:
Those solid air fresheners we often use in our cars rely solely on the magic of sublimation and thus, an example of sublimation. The solid fragrance within slowly transforms into a delightful vapour, filling the entire space with an enticing aroma that lingers in the air, just like the welcoming smell of spicy biryani.

Examples of Sublimation in Industries:
Applications of sublimation are also crucial to the function of several major industries. Who knew?
Fashion and Textile Design:
Sublimation breathes life into custom-printed clothing, giving rise to the vibrant threads of Pakistani attire. The intricate embroidery and rich motifs that we all fondly view in lehengas, dupattas, shalwar kameez, and sherwanis are all a result of sublimation. The ink sublimates into the fabric, creating stunning designs that have become part of our Pakistani identity.

Food Processing:
In the realm of food processing, sublimation examples are various as it is harnessed to freeze-dry an assortment of food items, such as fruits, vegetables, and our famous aromatic spices. This process not only prolongs shelf life but also safeguards the inherent flavours and nutritional goodness of these items.

Sports:
A prime sublimation example is Pakistan's sports equipment manufacturers. They employ the sublimation process to produce sports gear such as soccer balls and cricket bats with customized designs, logos, and branding.

Sublimation Example 7: Football design printing
Hope you enjoyed the applications and examples of sublimation as much as we did! 🙂 Here is an interactive video lecture on Sublimation to help you get the gist of the concept: