The intricacies of chemical bonding unveil a fascinating world of electrons with two primary protagonists: ionic and covalent bonds. In this comprehensive IGCSE and O Level Chemistry exploration, we'll unravel covalent and ionic bonds, and shed light on their fundamental aspects.
Ionic Bond
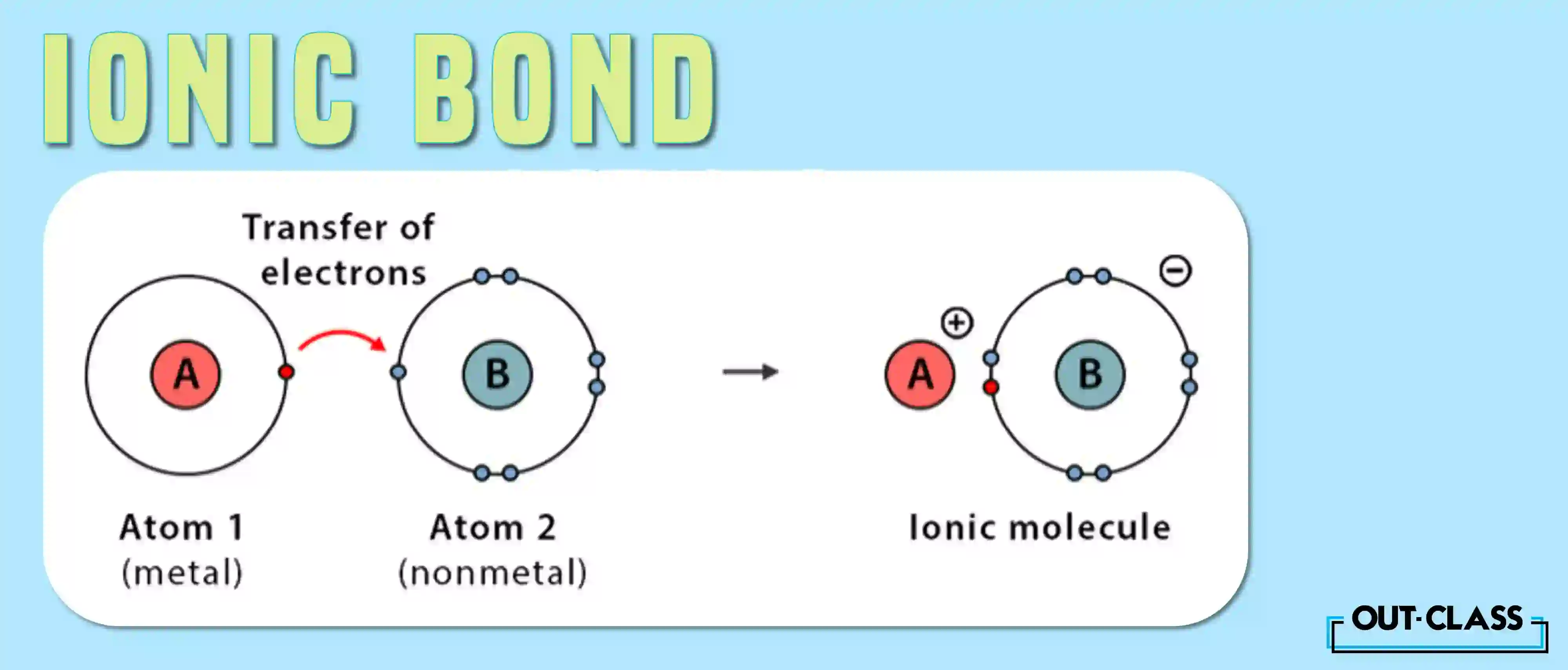
What is an Ionic Bond?
Ionic bonding revolves around the electrifying exchange of electrons.
What is the process of Ionic Bonding?
The process of ionic bonding entails a transfer from a metallic element to a non-metallic counterpart, resulting in the creation of cations and anions. Metals, generous in relinquishing electrons, become positively charged cations, while non-metals, adept at electron capture, transform into negatively charged anions. The endgame: both achieve a full outer shell, a state synonymous with stability.
Examples of Strong Ionic Bonds:
-
Sodium Chloride (NaCl)
-
Magnesium Oxide (MgO)
Covalent Bond
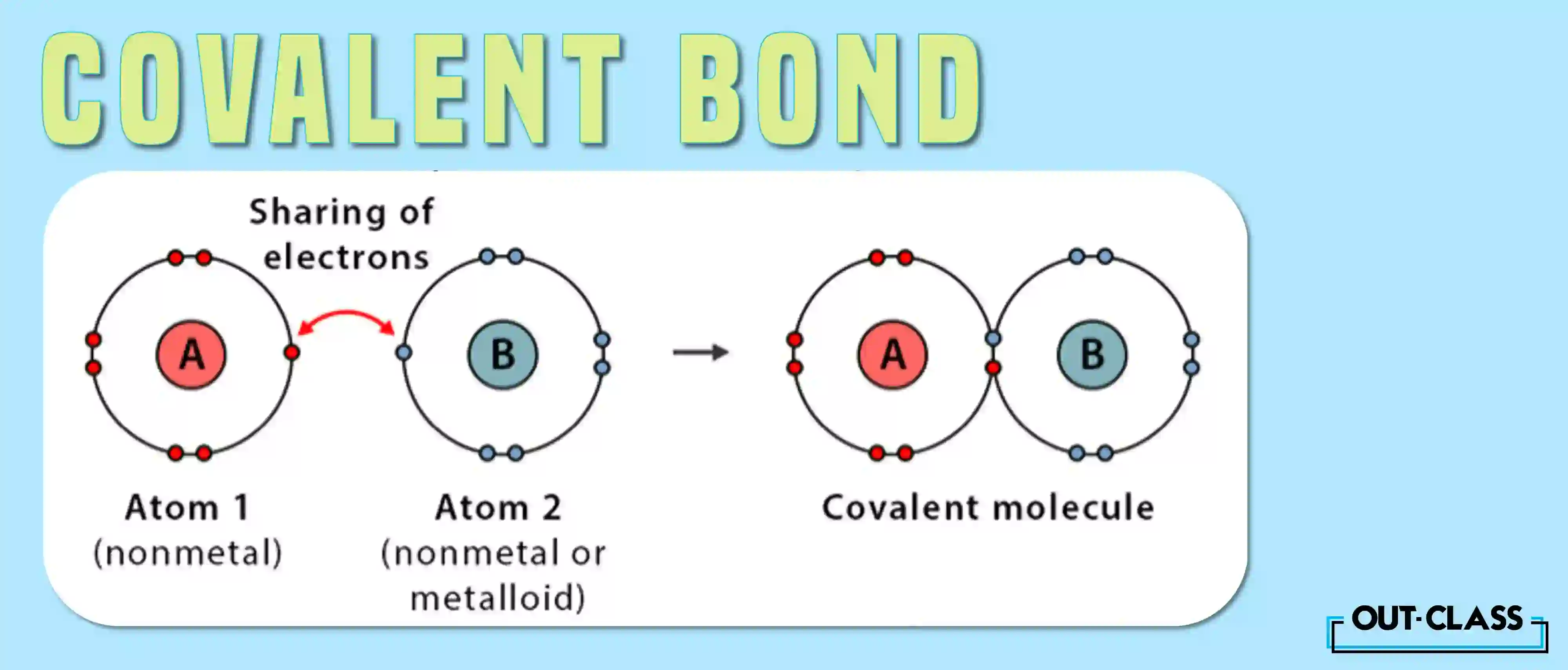
What is a Covalent Bond?
On the covalent frontier, atoms engage in an electron-sharing rendezvous, particularly among non-metals. Here, the objective is a noble pursuit of a full outer shell, and atoms achieve this by collaboratively sharing electrons in a bond.
Examples of Covalent Bonds:
-
Hydrogen Molecule (H2)
-
Carbon Dioxide (CO2)
Dative Covalent Bonds
Diving deeper into covalent bonding, we encounter the intriguing concept of dative covalent bonds. Unlike typical covalent bonds, where electrons are shared, a dative covalent bond involves a singular act — one atom donates both electrons in the shared pair.
Examples of Dative Covalent Bonds:
-
Ammonium Ion (NH4+)
-
Sulfur Hexafluoride (SF6)
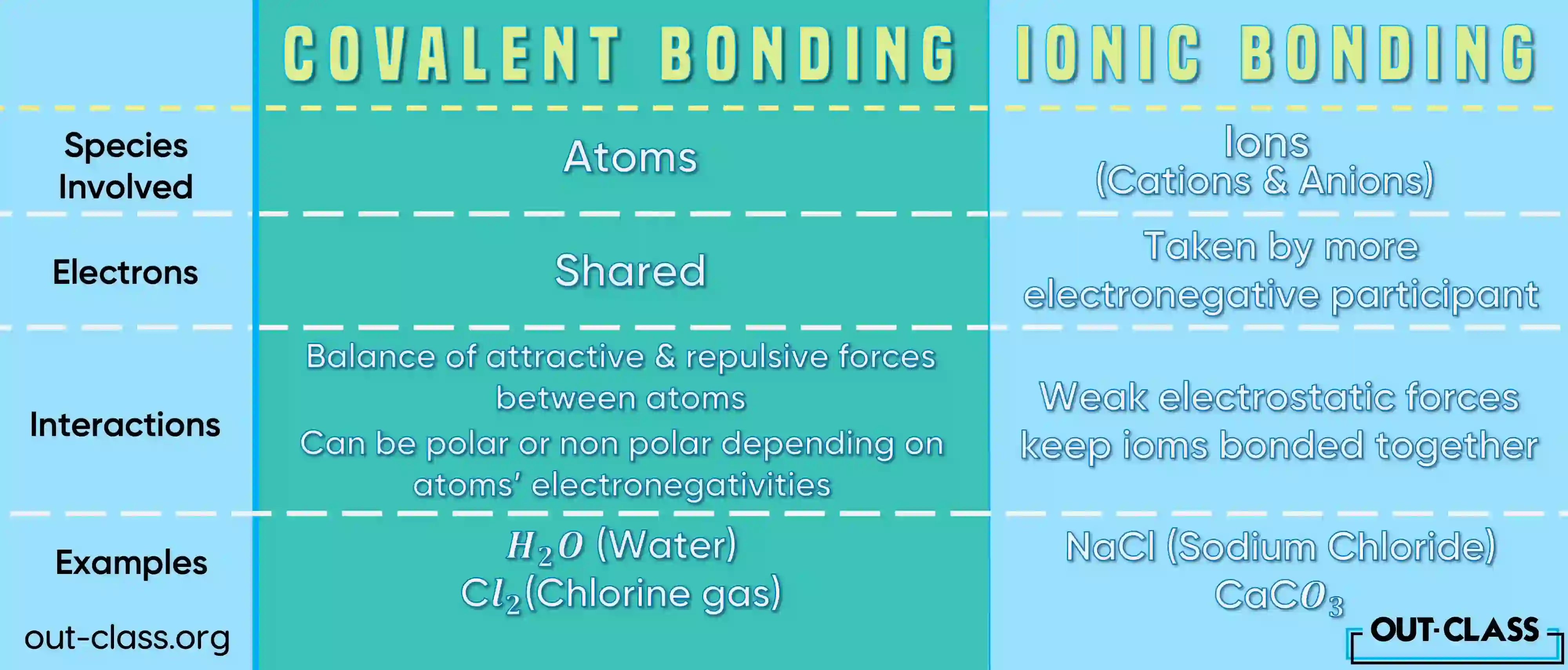
Which bond is stronger ionic or covalent?
The strength of ionic and covalent bonds can be compared based on various factors.
Ionic Bonds:
- Strength: Ionic bonds are generally strong.
- Nature: Ionic bonds involve the electrostatic attraction between oppositely charged ions (cations and anions).
- Melting and Boiling Points: Ionic compounds often have high melting and boiling points due to the strong electrostatic forces between ions.
- Solubility: Many ionic compounds are soluble in water.
- Conductivity: In a molten or dissolved state, ionic compounds can conduct electricity because ions are free to move.
Covalent Bonds:
- Strength: Covalent bonds vary in strength. Single, double, or triple bonds can form depending on the number of shared electron pairs.
- Nature: Covalent bonds involve the sharing of electrons between atoms.
- Melting and Boiling Points: Covalent compounds generally have lower melting and boiling points compared to ionic compounds. The strength of covalent bonds may depend on the number of shared electrons and bond multiplicity.
- Solubility: Covalent compounds can be soluble or insoluble in water, depending on their polarity.
- Conductivity: Most covalent compounds do not conduct electricity well because they do not produce free ions in solution.
Hence, the strength of a bond depends on various factors, and both ionic and covalent bonds can exhibit significant strength in different contexts. Ionic bonds are typically stronger in terms of energy required to break them, but the strength of covalent bonds can vary based on the specific atoms involved and the type of covalent bond (single, double, or triple).
Wrapping Up
As we navigate ionic, covalent, and dative covalent bonds in IGCSE and O Level Chemistry, the molecular tapestry becomes clearer. Ionic bonds showcase electron giving and taking, covalent bonds unveil the beauty of shared electrons, and dative covalent bonds underscore the generosity in electron donation.