Isotopes are integral to any Chemistry course (IGCSE and O Levels). Whether you’re coming across them for the first time or need to brush up on your concepts, here is a handy reference for you to go over!
What are Isotopes in Chemistry?
If you’re confused about how to define isotopes, here’s an easy isotopes definition as specified in the IGCSE and O Level syllabus:
Isotopes are atoms of the same element and have the same number of protons but different numbers of neutrons.
Physical and Chemical Properties of Isotopes
Having the same number of protons means isotopes of a given element have the same number of electrons. Since the chemical properties of an element are governed by electron configuration, we don’t notice many variations in these properties across isotopes. However, the different number of neutrons leads to a difference in atomic mass, which changes the physical properties between isotopes of the same element.
Example of Isotopes:
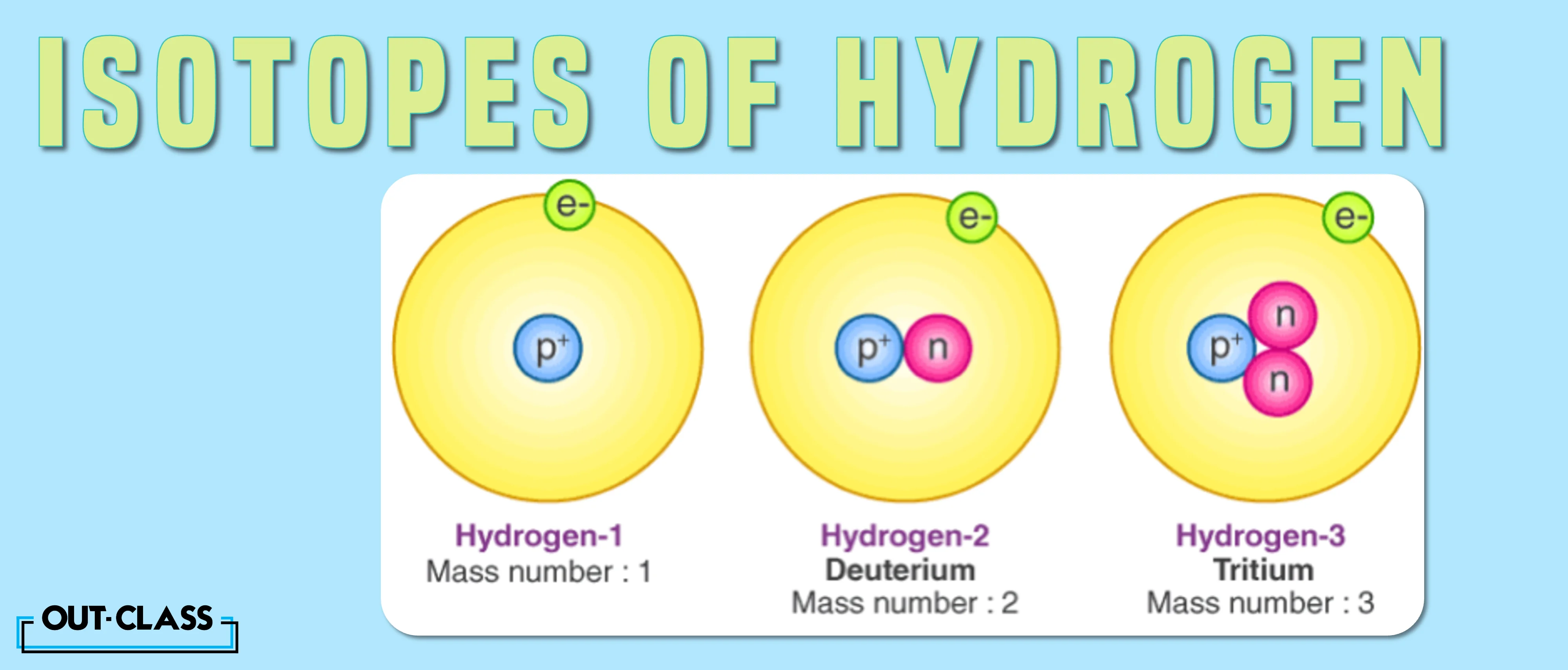
Isotopes of Hydrogen
As an example, let’s take Hydrogen, which has three isotopes:
-
Protium (1H)
-
Deuterium (2H)
-
Tritium (3H)
Whereas protium and deuterium have similar chemical properties, water made from heavier deuterium isotope (D2O) has a higher melting and boiling point than water made from protium (H2O).
What are the Stable Isotopes of Carbon?
Whereas 12C and 13C are stable, 14C belongs to the class of radioactive isotopes. Similarly, tritium is also radioactive, emitting beta particles!
Why are Isotopes important in Chemistry?
In answering the question, “What are isotopes in Chemistry”, we must understand how to use their properties. A prime example is 14C, used by scientists in “carbon dating”. Since plants utilize carbon to build molecules, the small fraction of radioactive 14C buildings in them can trace how old plant fossils are.
If you found this guide helpful, please check out the IGCSE/O Level Chemistry crash course here that can help you prepare for success in your exams!