Yikes, the burning point of alcohol, right? Well, let me tell you, it’s not that simple. There are many kinds of alcohol, and each one has a different boiling point.
For example, the alcohol that people drink is called ethanol, and it boils at around 78 degrees Celsius. That’s pretty hot, but not as hot as water, which boils at 100 degrees Celsius. Do you have some clarity about what temperature alcohol boils at?
Why is that? It’s because ethanol and water are polar molecules, which means they have opposite charges on their ends. They just like to stick together with other polar molecules, and that makes them harder to separate. This simple mechanism is called hydrogen bonding, and it’s a powerful force. So, to answer the question is alcohol non-polar, the answer is yes.
Ethanol Boiling Points
However, the boiling point of ethanol can change depending on other factors, too. For example, if you add salt or sugar to water, you will change the alcohol's boiling point.
So what temp does ethanol boil at? The boiling point of alcohol in Celsius is 78.37 °C.
Salt will make it boil at a higher temperature, while sugar will make it boil at a lower temperature. Alcohol boiling points can change. This is because salt and sugar change the number of water molecules in the solution, and that affects how easily they can escape as vapour. Alcohol boiling points are not that complex.
Alcohol Evaporation Point
The more vapour there is, the easier it is to boil. The less vapour there is, the harder it is to boil.
When the vapour pressure of a liquid is equal to the air pressure, the liquid boils. We hope that clarifies alcohol boiling points more.
The rate of evaporation depends on many things, such as the surface area of the liquid, the temperature of the liquid and the air, the humidity of the air, and the wind speed. The evaporation point of ethanol is not a fixed value, but it is generally lower than its boiling point because evaporation does not need the liquid to reach its vapour pressure.
The Burning Point of Alcohol
Another thing you should know about ethanol is that it can catch fire very easily — the burning point of alcohol.
The lowest temperature at which it can form a flammable vapour is called the flash point, and for ethanol, it’s only 13 degrees Celsius. That means that ethanol can burn even when it’s not boiling. But it doesn’t mean it will burn at any temperature above 13 degrees Celsius. It also depends on how much ethanol there is in the air, how much oxygen there is, and what kind of spark or flame there is. For ethanol to burn, it needs to have a concentration between 3.3% and 19% in the air, and it needs to have enough oxygen and heat to ignite the vapour.
Conclusion:
Alcohol's boiling point varies by type; ethanol, the kind in drinks, boils at about 78°C due to its polarity and hydrogen bonding with water. Adding salt or sugar changes the boiling point. Ethanol can ignite at a low flash point of 13°C, but combustion depends on concentration, oxygen, and heat. If you found this information helpful, imagine what an entire Chemistry course can do for you. Digest any complex topics, within minutes using Out-Class’ top-notch IGCSE/O Level lectures which guarantee results and secret hacks for O Level success.
Most Common Repeated Questions:
Unlock the secrets to acing your CAIE IGCSE/O Level exams with a sneak peek into the most frequently asked questions that have graced the pages of past papers!
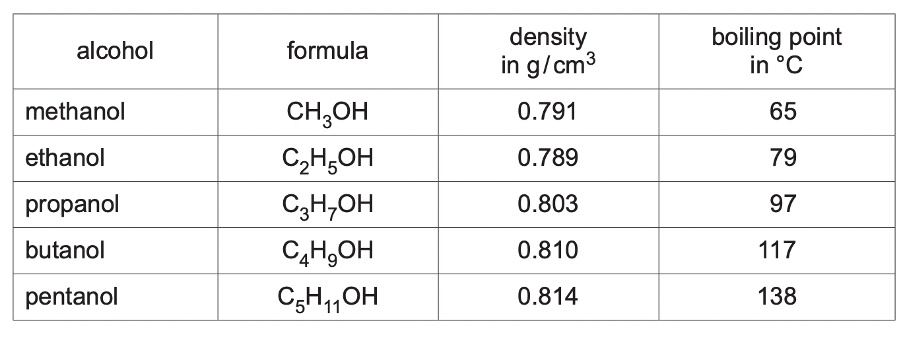
- The table shows some properties of five alcohols. Describe and explain the change in the boiling point of the alcohols as the number of carbon atoms in a molecule increases. (1) [Oct/Nov 2020]
FAQs
Q. What is the boiling point of ethanol?
The boiling point of ethanol in Celsius is approximately 78.37 degrees Celsius.
Q. Is ethanol a polar or nonpolar molecule?
Ethanol is a polar molecule due to its structure, which contains an oxygen-hydrogen (OH) bond, resulting in opposite charges on its ends.
Q. How does the addition of salt or sugar affect the boiling point of ethanol?
Adding salt or sugar to ethanol and water mixture can change its boiling point. Salt increases the boiling point, while sugar lowers it. This is because they affect the number of water molecules in the solution.
Q. What is the flash point of ethanol?
The flash point of ethanol, the lowest temperature at which it can form a flammable vapour, is around 13 degrees Celsius.
Q. Can ethanol catch fire below its boiling point?
Yes, ethanol can catch fire even below its boiling point. Its flash point is 13 degrees Celsius, indicating that it can ignite at temperatures lower than its boiling point under the right conditions.
Q. What factors influence the rate of evaporation of ethanol?
The rate of evaporation of ethanol is influenced by factors such as the surface area of the liquid, temperature of the liquid and air, humidity, and wind speed.
Q. Why does ethanol have a lower flash point compared to its boiling point?
The flash point is the lowest temperature at which ethanol can form a flammable vapour. It is lower than the boiling point because evaporation, which precedes boiling, does not require the liquid to reach its vapour pressure.
Q. What concentration of ethanol in the air is required for combustion?
Ethanol needs a concentration between 3.3% and 19% in the air for combustion. The presence of enough oxygen and heat is also necessary to ignite the vapour.



